What is the reason behind choosing "Lanthanum doped with europium"?
Well, you said it yourself, because "fluoride ions bind to the crystal".
Here's a great explanation from Wikipedia:
In the lanthanum fluoride electrode, the sensing element is a crystal of lanthanum fluoride LaF3, doped with europium fluoride EuF2 to create lattice vacancies. Such a crystal is an ionic conductor by virtue of the mobility of fluoride ions which jump between lattice vacancies.
Here's a rough sketch I made to help you visualizing this. This is a simplistic drawing and the actual crystalline structure of the compound is 3-dimensional.
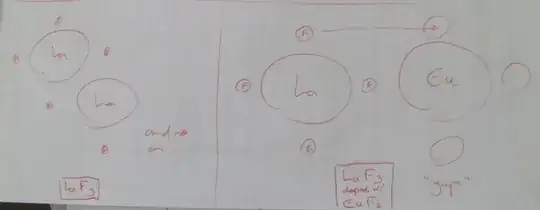
I must stress this: Both the structure and the proportions are probably nowhere near the ones in my drawing, it is only for didactic purposes.
An electrochemical cell may be constructed using such a crystal as a membrane separating two fluoride solutions. This cell acts as a concentration cell with transference where the fluoride transport number is 1. As transference of charge through the crystal is almost exclusively due to fluoride, the electrode is highly specific to fluoride. The only ion which significantly interferes is hydroxide (OH-). Generally such "alkaline error" can be avoided by buffering the sample to a pH below 7.
We had the pH around 5.5. We also used TISAB for the preparation of water samples. The Wikipedia article on TISAB gives an excellent explanation on it's usage in fluoride ion analysis.
Why ASP cannot be used for determining the concentration of anions?
Well, I cannot give you an authoritative answer on that. I'll ask a teacher of mine this week and edit the answer with an update - if someone reading this knows it feel free to improve this answer or add a new one. In the case of fluoride it's probably because the Lanthanum electrode has pretty decent detection limits (it does). It's a lot cheaper to use it instead of AAS.
