Since not many people know the ligand formazanate (I hadn’t heard of it before this question), let me quickly supply an image of what it looks like:

Scheme 1: General formazane structure.
Coodination happens from the lower two nitrogens, one of which is deprotonated (hence -ate) and both of which chelate the metal with $\mathrm{sp^2}$-type orbitals. The five formazane atoms form a π system, which can be extended by appropriate $\ce{R}$-groups. And one of these $\ce{R}$-groups is now modified to include an electron-withdrawing group.
I already previously gave a qualitative orbital scheme for a tetrahedral complex including σ and π interactions as given below.
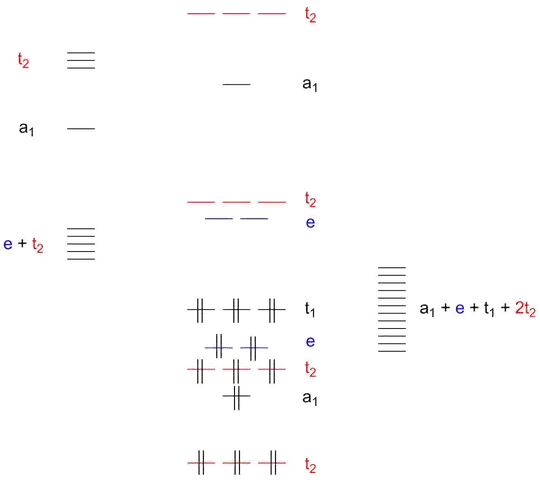
Figure 1: Qualitative MO-scheme of a tetrahedral $\mathrm{d^0}$ complex with σ and π interactions. Originally created for this answer.
The σ donor orbitals transform according to $\mathrm{a_1 + t_2}$, leaving $\mathrm{e + t_1 + t_2}$ for the π ligands. Unfortunately, the picture is a tad clouded, since both σ and π interactions have a $\mathrm{t_2}$ contribution.
Adding an electron-withdrawing group to your ligand will leave the σ interaction unchanged in first approximation, since mainly the ligand’s π system would be affected. The orbital energies of the π system would be reduced. Since we are talking about a metal with a low oxidation state, its orbitals are higher than drawn in the qualitative scheme (which was designed for permanganate). And thus also, the most important π interaction of the ligand will be, as you stated, accepting electrons into its π system.
The exact nature of any effect observed will depend on the original orbital energies. However, the $\mathrm{e}$ orbitals are only affected by the π interaction while the $\mathrm{t_2}$ orbitals are also affected by the σ interaction. The magnitude of π interaction is expected to be the same for both groups of orbitals, since the start from the same value on each side. Thus, the difference between $\mathrm{e}$ and $\mathrm{t_2}$ stems mainly from σ interactions which only affect $\mathrm{t_2}$ due to symmetry.
Because the σ interaction starts with the ligand orbitals being much lower in energy than the metal orbitals, reducing their energy even more will result in a weaker interaction, therefore a lowering of the $\mathrm{t_2}$ level and thus a smaller $\Delta_\mathrm{T}$ or $10~\mathrm{Dq}$ value.

