I am looking for a simple way to distinguish between glucose and fructose samples. Currently, I am doing that by melting temperature method. Can anybody suggest me some easy way to do that?
-
1Related: Why does fructose reduce Tollen's reagent and Fehling's solution? – Aug 15 '16 at 10:58
4 Answers
One of the simplest ways would be bromine-water test. Bromine water oxidizes glucose to gluconic acid, hence decolorize the solution. Being a mild oxidizing agent, Bromine water is not capable of oxidizing fructose (ketone).
- 371
- 1
- 6
Go for Seliwanoff's Test. It is based on the simple fact that when heated, ketose sugars are more rapidly dehydrated than aldoses. After acidic hydrolysis of both, we add a pinch of resorcinol(0.5%) and concentrated HCl(3N). Fructose reacts to give a deep red cherry colour whereas Glucose reacts slightly to produce a faint pink colour.
- 21,621
- 5
- 38
- 89
- 41
- 2
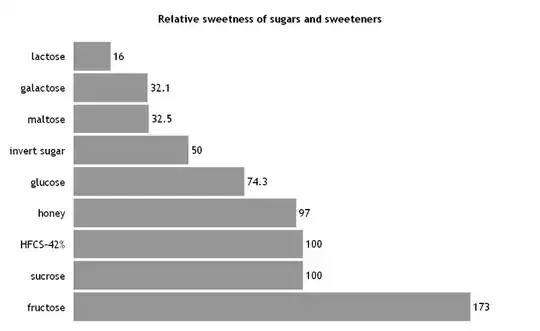
Taste tests also work. Fructose is about 2.3 times sweeter tasting than glucose, and the tongue is a very good sweet-sensor.
(source: wikipedia)
- 1,556
- 10
- 12
-
7While this is accurate, normally one does not recommend tasting chemicals in a laboratory. – Lighthart Aug 15 '16 at 19:43
Go for Osazone test.... add phenylhetrazine +sodium acetate + acetic acid...if yellow precipitate appears within two minutes of boiling in waterbath (needle shape crystals)then the sample is Fructose.. if yellow precipitate appears between 5 to 10 minutes of boiling in waterbath (broom shaped crystals)its glucose.
- 11
- 3