I know that $\ce{OH-}$ is usually a bad leaving group, but why does the following reaction occur via SN1 in presence of anhydrous $\ce{ZnCl2}$?
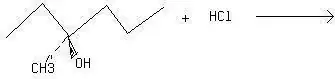
I know that $\ce{OH-}$ is usually a bad leaving group, but why does the following reaction occur via SN1 in presence of anhydrous $\ce{ZnCl2}$?

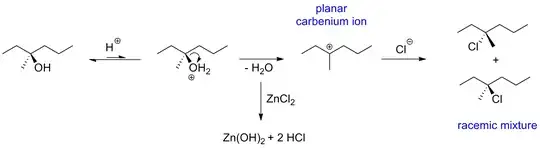
You are right, $\ce{OH-}$ is a bad leaving group. But the $\ce{HCl}$ in the reaction mixture protonates it. Thus, the bad leaving group $\ce{OH-}$ is converted to the good leaving group $\ce{H2O}$. Anhydrous $\ce{ZnCl2}$ might additionally facilitate the reaction by acting as a Lewis acid and it binds the expelled water thus preventing the back reaction.