My teacher had once told me that graphite was just an insanely huge polymer of benzene (Those weren't his exact words but the gist). Today While we were studying aromaticity in organic compounds we steadily advanced from benzene to napthalene the annulenes and so on. What really stuck me was how as the number of benzene rings increased the stuff tended towards graphite (atleast thats what the structure seemed to indicate). So the main question is why isn't graphite aromatic if its just a big collection of benzene molecules
Asked
Active
Viewed 4,786 times
1 Answers
11
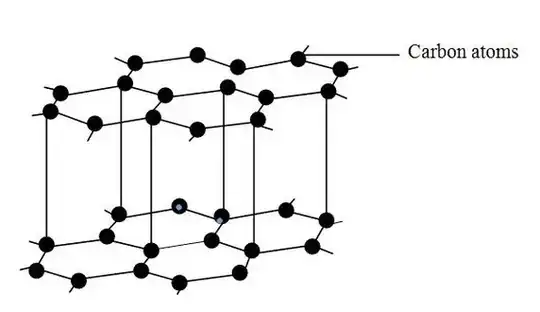
Graphite/graphene is aromatic. The first image below is a common crystallographic representation of graphite and does not account for covalent bonding. The second one shows the molecular structure of graphite as a kekule representation. Graphite is made of planar sheets of carbon atoms in 6-atoms rings with conjugated double bonds. This also satisfies the 4n+2 π electrons criteria needed within the aromatic ring for graphite to be aromatic. This aromaticity is why graphite is not only stable to high temperatures but also a more thermodynamically favorable allotrope than diamond under typical conditions.
A.K.
- 12,460
- 7
- 45
- 93

+1(possibly helpful http://ion.chem.usu.edu/~boldyrev/graphene.php) and so I've just asked How is the aromaticity in graphene different from the aromaticity in benzene? – uhoh May 13 '19 at 00:57