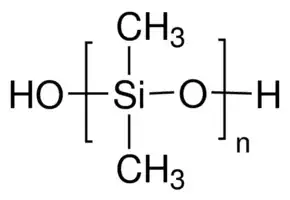
Consider poly(dimethylsiloxane) (PDMS), a member of the family of silicone polymers:
Now, imagine its carbon analogue:
I've not been able to find any evidence that this carbon analogue has been experimentally prepared. Searching for "poly(dimethylalkoxane)" got me nowhere. I eventually searched for "dimethyl polyoxymethylene," which turned up the unsubstituted variant of the above, polyoxymethylene (POM):
as well as methyl-capped POM oligomers, polyoxymethylene dimethyl ethers:
But, no "poly(dimethyloxymethylene)" was forthcoming.
Has the carbon analogue of poly(dimethylsiloxane) been synthesized? If so, what name has it been given?