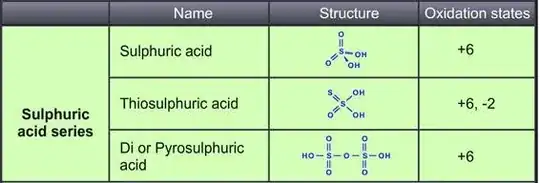
I) According to my logic, sulfur-sulfur linkage (linkage between same atoms) result in no charge separation.So, the oxidation number of the sulfur atom linked to only sulfur should be 0(zero) and other sulfur being +4 as it is connected to 3(more electronegative) oxygen atoms.
Thus, the oxidation number of sulfur atoms should be +4,0.
II)However, Using X-ray absorption to probe sulfur oxidation states in complex molecules claims that oxidation states are +6 and -2.
III)Again,Determination of Changes in Sulfur Oxidation States in Prostate Cancer Cells proved that that oxidation states are +5,-1.
These three sets of results left thoroughly confused.Which one of them is correct and why?
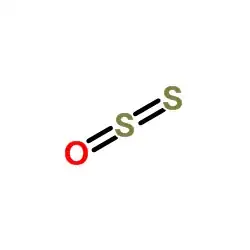
Following the same sequence of logic: My counter-question for the claims (I) and (II) is "Why the oxidation number of sulfur atoms of Disulfur monoxide is [ +2 and 0 ] instead of [+4 and -2] or [+3 and -1] ?"