When water temperature reaches $100\ ^\circ \mathrm{C}$, the molecules get so excited that the hydrogen atoms lose the bonds to the oxygen atom and therefore the water starts to become gas. I get that, but at room temperature ($23\ ^\circ \mathrm{C}$), is there no excitation in the atoms or is there?
2 Answers
First, I think I should make it clear that when water boils, the bonds in the water molecule linking the hydrogen and oxygen atom are not broken. During boiling, the intermolecular bonds in water are the ones that get broken, that is the bonds that link the water molecules together.
At room temperature, there is evaporation (I wouldn't call it excitation). This is because there are a few molecules of water which can manage to muster enough energy to escape from the large body of molecules and escape into air.
This can be explained through a graph depicting the distribution of speed among water molecules worked out by Maxwell and Boltzmann.
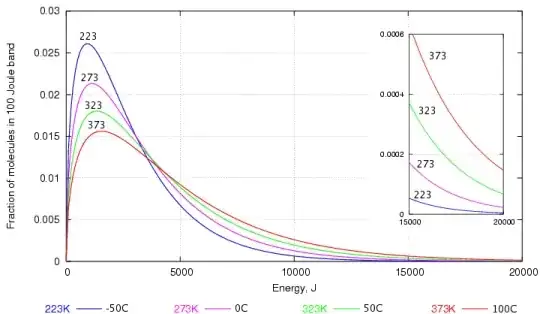
As you can probably see, there are a lot of water molecules with lower kinetic energy than with higher kinetic energy. Those that have the higher kinetic energy are the ones that are able to break through the water surface to become vapour.
Even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can occur at any temperature (yes, even if the water is in ice).
When the temperature increases, there are more molecules with higher kinetic energy and thus, more water can evaporate.
- 2,083
- 20
- 24
-
@Kelpie Yes, they are still $\ce{H2O}$. As for whether they are never separated or not, I would say that you will need a lot lot more of energy to break the $\ce{H-O}$ bond with heating alone. You can however have a reaction where the energy released is enough to overcome the energy stored in the $\ce{H-O}$ bond. – Jerry Dec 21 '13 at 10:02
-
Wow, I'm curious as to what drove the sudden few upvotes lol. Thanks guys! – Jerry Nov 10 '14 at 19:35
-
1Although you explain this properly your graph is for molecules on the gas phase, not solution. Also the abscissa is presumably in joules/mole? The Boltzmann distribution $ exp(-\Delta E/RT)$ is probably more appropriate, it shows the same effect except at low energy. – porphyrin Jul 20 '16 at 08:54
-
1Reading through this, it doesn't seem to explain how a puddle can completely evaporate, even in freezing temperatures. Extrapolating from "few" and "some" to "all" isn't described. – whatsisname Aug 12 '20 at 20:21
-
@whatsisname I think I didn't add that because that was not the question. Is that something you want to ask about or is it more about something you think should be added to the answer? – Jerry Aug 13 '20 at 11:08
-
@Jerry I think it's part of the question, if 30% of the molecules are high energy enough to escape, then your average pool of water would shrink to 70% of its size and then no further evaporation would occur. So there must be a little bit more to the process. – NibblyPig Mar 15 '22 at 12:57
-
1@NibblyPig I'd argue that it can be a different question on its own (the OP was about how having evaporation at all at rtp). If you want an answer to that though, it'll have to do with heat transfer from the surroundings. When evaporation occurs, the water molecules left behind have lower average energy (i.e. colder) and thus there is increased heat transfer from the surroundings through conduction. When that occurs, you're back at the previous situation and evaporation can continue occurring at the same rate before taking other variables like surface area and pressure into consideration – Jerry Mar 18 '22 at 04:33
To add to Jerry's answer, the amount of evaporation of water also depends on pressure.
Infact, one way of defining boiling point is the temperature at which the vapour pressure equals atmospheric pressure. So, you can actually boil water at room temperature.
- 709
- 1
- 5
- 11