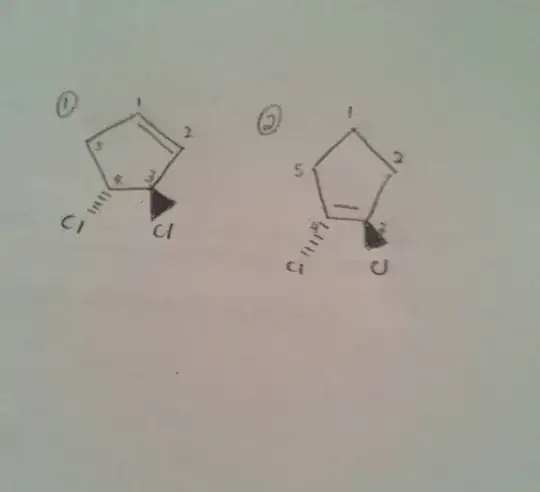
The issue here really is that the name 'trans-3,4-dichlorocyclopentene' is not really correct, as it is unspecific. It lacks stereo-descriptors, and the locant for the double bond.
Assuming that the double bond starts at the first carbon, which is usually the case if it is omitted, there are two enatiomers still possible:
(3R,4R)-3,4-Dichlorocyclopent-1-ene
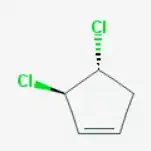
See for details: National Center for Biotechnology Information. PubChem Compound Database; CID=12430197.
(3S,4S)-3,4-Dichlorocyclopent-1-ene

See for details: National Center for Biotechnology Information. PubChem Compound Database; CID=131015828.
So the structure you have drawn as 1 is (3R,4R)-3,4-dichlorocyclopent-1-ene.
The structure you have drawn as 2 doesn't really exist, as the double bond is planar, but ignoring that it would be 1,2-dichlorocyclopent-1-ene.
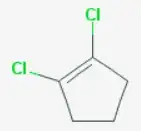
See for details: National Center for Biotechnology Information. PubChem Compound Database; CID=10606793.