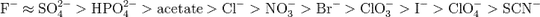A similar effect occurs in synthetic polymers. poly(N-isopropyl Acrylamide), (usually just p(NIPAM)) is a thermosensitive polymer. At room temperature, it dissolves easily in water. When you heat the solution past a certain temperature (the "lower critical solution temperature," or LCST) the polymer chains undergo a conformational change and precipitate out of solution. When you cool the solution back down below the LCST, the polmer returns to its original conformation and re-dissolves.
http://pubs.acs.org/doi/abs/10.1021/jp0690603 and http://pubs.acs.org/doi/abs/10.1021/ja0546424 are two papers from a group at Texas A&M that show that the Hofmeister series applies to these thermosensitive polymers in a similar manner. In this case, the ions lower the LCST. So even at room temperature, you could salt out the polymer with enough salt. The Hofmeister series indicates the efficacy of the ions in this salting out.
Those papers divide the ions in the Hofmeister series into "chaotropes" and "kosmotropes," depending on whether the ion tends to break apart the natural structure of the water, or tends to stabilize the natural structure of the water. They claim that the chaotropes operate by changing the surface tension of the water and by direct anion binding to the polymer, and that the kosmotropes operate through surface tension and by changing the polarization of the water molecules. They correlate the magnitude of the effect of each ion on LCST with that ion's hydration entropy and surface tension increment.
Caveats: I'm only really comfortable with p(NIPAM) itself, and with the empirical fact of the salt effect. I don't know how well-understood these entropies of hydration or surface tension increments are, or if this would apply to salting-out of proteins.