I want to make space filling model for nitrogen molecule.
I've learned that van der Waals diameter is to be taken for atom radius.
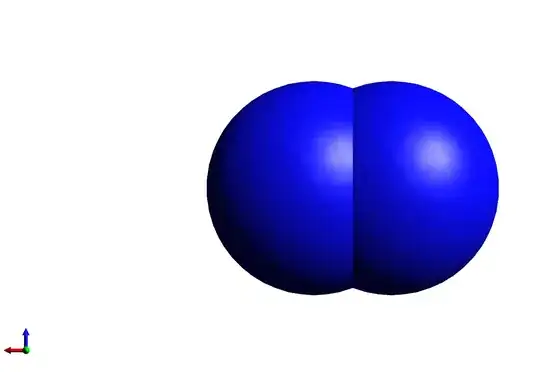
Since van der Waals radius for nitrogen is $\pu{155 pm}$ and bond length is $\pu{110 pm}$, does this mean that nitrogen molecule space filling model is two blue spheres of diameter $\pu{155 pm}$ and center to center distance $\pu{110 pm}$?
One of the reasons for this question is that samples of space filling model for this molecule (see for example Wikipedia) do not agree with these data; there's about a 11% difference between the length/width ratio of the drawn molecule, and what it should be based on the van der Waals radius and bond length.
\begin{array}{cccccc} \displaystyle\frac{d}{2r} &\sim& \displaystyle\frac{\pu{1000 pix}-\pu{716pix}}{\pu{716 pix}} &\sim& 0.397\\ \displaystyle\frac{d}{2r} &\sim& \displaystyle\frac{\pu{110 pm}}{2\times\pu{155 pm}} &\sim& 0.355 \end{array}

\cdotto\times, because sometimes that looks like a decimal delimiter on some devices.) [Flag this comment obsolete when read ;)] – Martin - マーチン Jul 24 '17 at 10:53