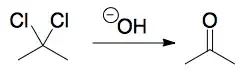
How does nucleophilic substitution work in a compound consisting of two leaving groups?
I understand that hydroxide ions will attack the carbon centre (which bears a partial positive charge) and cause a nucleophilic substitution reaction.
What I'm unsure of is whether the reaction proceeds via
The hydroxyl groups replacing the leaving groups one at a time (double $\mathrm{S_N2}$).
After the replacement of the first hydroxyl group, the repulsions between the electron pairs of oxygen and of chlorine cause the chlorine, a better leaving group, to leave, causing formation of a cation. ($\mathrm{S_N2}$ followed by $\mathrm{S_N1}$).
Both bonds forming at once (a transition state with six bonds to the center), although this seems very unlikely.