The answer that you are supposed to give is presumably something along the lines of "dπ–pπ" bonding between silicon and nitrogen. This essentially means that the lone pair of nitrogen is involved with backbonding into a silicon 3d orbital, leading to a linear geometry at nitrogen (i.e. the bond angle is 180°). This can be represented with the following resonance structures:
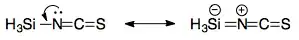
To be very clear, this is very likely incorrect. The extent of d-orbital involvement in main group compounds is usually tiny. If there is any backbonding, the acceptor orbital is probably a Si–H σ* orbital rather than a d-orbital (cf. backbonding from transition metal to phosphine).
Electron diffraction studies of H3SiNCS show that the Si–N–C bond angle is actually 163.8°.1 A computational study (TZVP + MP2 level of theory) found 165.91°.2. In fact, the linear structure above is not an energy minimum, but rather a transition state for the interconversion of the two energy minima:3

I don't actually know how one can predict this a priori without access to a computer, but clearly this system is not amenable to bond angle prediction with VSEPR theory, and invoking the so-called "Si–N dπ–pπ bonding" doesn't give the correct answer either.
References
Glidewell, C.; Robiette, A. G.; Sheldrick, G. M. Electron diffraction study of large amplitude vibrations in H3SiNCO and H3SiNCS. Chem. Phys. Lett. 1972, 16 (3), 526–529. DOI: 10.1016/0009-2614(72)80416-0.
Palmer, M. H.; Nelson, A. D. The structures of the azido-, isocyanato- and isothiocyanato-derivatives of methane and silane and their derivatives. A comparison of ab initio with experimental results. J. Mol. Struct. 2004, 689 (1–2), 161–173. DOI: 10.1016/j.molstruc.2003.11.008.
Palmer, M. H.; Guest, M. F. The methyl, silyl and germyl esters of the pseudo-halogen acids. A comparison of structural data from experimental and ab initio studies. Chem. Phys. Lett. 1992, 196 (1–2), 183–190. DOI: 10.1016/0009-2614(92)85951-6.


