If I were to rotate the front carbon clockwise by $60^\circ$, it would become achiral. But my text book says that it's chiral. Is it that rotating an individual atom about a sigma bond changes its conformation is that why we can't rotate it?
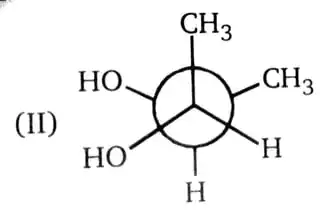


If I were to rotate the front carbon clockwise by $60^\circ$, it would become achiral. But my text book says that it's chiral. Is it that rotating an individual atom about a sigma bond changes its conformation is that why we can't rotate it?



It simply depends on what is the subject. The book asks for the displayed conformation, which we agree is chiral.
The question would have been more tricky to answer if it were about optical activity of a sample.
In this case we know the conformer can not be isolated at room conditions but this is not a general rule.
And one can always cool down, at least in principle.