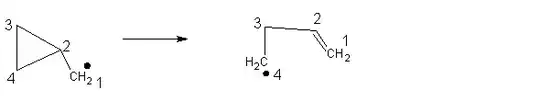
Ring opening of the cyclopropylmethyl radical is reliable and fast, and so has been used as a radical trap and a radical clock.
Could someone please explain what exactly makes this reaction so fast, besides the ring strain? Why is the anionic ring opening slower?