Is it a co ordinate covalent bond,I think that due to high electronegativity of the fluorine atom it will attract the hydrogen electron towards itself.Am I right in thinking so?
1 Answers
The bond between $\ce{H}$ and $\ce{F}$ atom in $\ce{HF}$ molecule is a polar covalent bond.
Though the electronegativity of $\ce{F}$ is sufficiently high, but $\ce{H^+}$ is a tiny ion with a positive charge on it, which creates high electronic charge density on the atom and due to its much smaller radius,The polarising power ($\phi$) becomes very high. So, according to Fajan's rule, there is a significant covalent character.
If you want to think in terms of valence bond theory, then $1s$ orbital of $\ce{H}$ containing a single electron pairs with one of the $2p$ orbital of $\ce{F}$ containing a lone electron to form a covalent bond.
Similarly, if you consider molecular orbital theory, the molecular orbital diagram will be something like this,
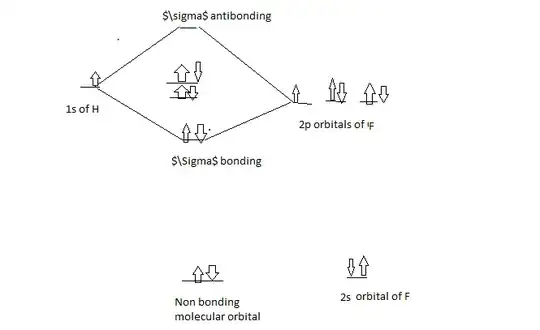 So, the bonding electron resides in a $\sigma$ bonding orbital, which indicates there is a covalent bond.
So, the bonding electron resides in a $\sigma$ bonding orbital, which indicates there is a covalent bond.
- 3,316
- 2
- 16
- 34
- 5,688
- 1
- 16
- 33