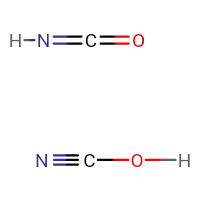
What anions does the loss of a proton from $\ce{HOCN}$ and $\ce{HNCO}$ produce? Are they same or different?
Once, I had asked my teacher if $\ce{HCN}$ and $\ce{ HNC }$ are different or same compunds, he said they were different and yes, they had different structures. Applying the same logic here, $\ce{OCN-}$ and $\ce{NCO-}$ are different anions because they'll have different structures.