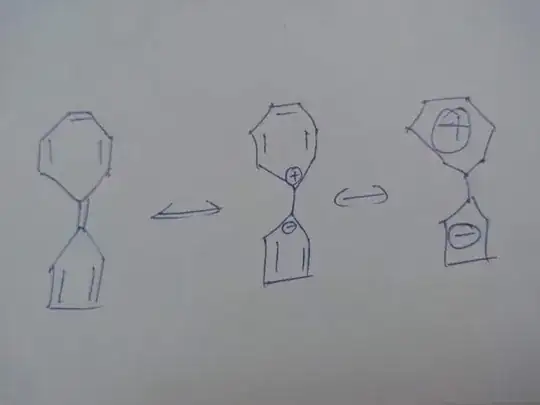
So for this question I'm considering a chemical to be "similar to azulene" if it obeys the following conditions:
- It is aromatic and obeys Hückel's rule,
- It is a fused bicyclic compound,
- One component ring is aromatic as an anion,
- The other ring is aromatic as a cation,
- It is not azulene, and
- It has been synthesised before and the synthesis has been confirmed.
For instance, I believe bicyclo[3.1.0]hexa-1,3,5-triene should follow all of the previous conditions except possibly the last (but I don't know that it hasn't). Do any chemicals obey all of these conditions?