This tag should be applied to questions about the layout, history and interpretation of the periodic table, not to questions relating to specific elements or trends within the table. For these, the tags 'elements' or 'periodic-trends' should be used where appropriate.
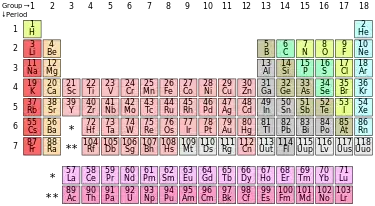
The periodic table is a systematic arrangement of the known chemical elements in order of atomic number. The elements are arranged in groups on the basis of similarity in chemical and physical properties.
Above is the standard periodic table used by most scientists around the world. However, alternatives exist, ranging from useful ones which order the elements based on electron configuration, to artistic designs which are purely aesthetic in their appeal.