I am trying to solve the following iterative equation:
where the parameter tao (needed in the previous equation) is defined as:
Here's the parameters and my approach to solving the equation in Mathematica:
ah = 342496; (*J/mol*)
R = 8.314; (*J/mol.K*)
A = 7.6*10^-38; (*s*)
b = 0.67;
x = 0.49;
q = -0.1/60;(*K/s*)
T0 = 500;(*K*)
Tfinal = 350;(*K*)
dt = 0.1 ;(*s*)
n = (T0 - Tfinal)/dt; (*number of steps*)
Tf0 = T0;
dT = dt*q; (*K*)
Tk = T0 + Sum[dT, {i, 1, n}];
τ = A*Exp[(x ah)/(R Tk) + (1 - x) (ah/Subscript[T, fkminus1])];
Tf = T0 + Sum[dT (1 - Exp[-Sum[(dT/(q*τ))^b, {i, 1, n}]]), {i, 1, n}]
In the code, notice that dT = dt*q, where dt is a time step that I choose which ideally should be small enough as to ensure that Tf decays less than 1 K. Also, notice that $\tau_{0,k}$ is a function of $T_{f,k-1}$ ($T_{f,0}$ is defined in the code as 500) and $T_k$.
The problem I am having is that I do not know how to connect the two equations so that the $T_k$ that I calculate from equation 1, eventully goes to equation 2 and the $\tau_k$ that I calculate from equation 2 goes to equation 1.
Questions
- How can I solve the Tf equation?
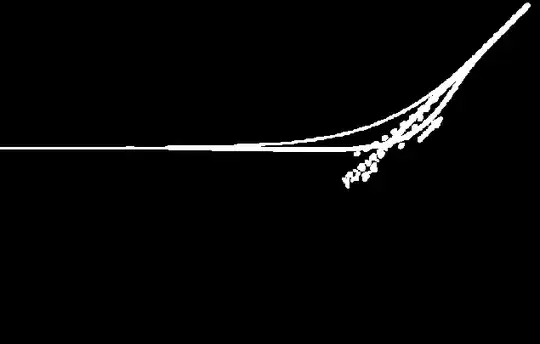
- How can I plot Tf vs T, similar to the figure below (only for one q, in this case for
q = -0.1/60)?. Notice that the parameters from figure below are the same I am using in the code.
Note: Here's the paper where the two equations are coming from as well as the figure (figure 1 in that paper) in case anybody wants to look at it: https://sci-hub.se/10.1016/s0260-8774(02)00212-1.
I hope everything is clear and I appreciate in advance your help.
EDIT: Clarification on the parameters
ΔTis the change in temperature and it is related by the change in time (which is set by the user, small enough so that Tf doesn't decrease more than 1 K) asΔT=dt*q. Both, dt and q can be taken as constant.ΔTkrepresents then a series of temperature steps.qkis the cooling rate in Kelvin/seconds at which the process is performed. In the code it fixed with value of -0.1/60 (or -0.0016 Kelvin/second). The negative sign is because it is a cooling process (it would be positive if it was heating).T,kis simply the temperature, which is defined asTk = T0 + Sum[dT, {i, 1, n}], whereT0is the initial temperature of the process and is 500 (kelvin).Δhis an apparent activation energy in J/moltao,k(from equation 20) is the relaxation time and it is a function ofTf,k-1(The initial Tf(0)=500) andTkTf,kwhich is what I want to calculate can be considered to be as the "glass transition temperature". Hence, it depends on the material and it is quite different thanT,kwhich is just the process temperature.
So, as you can see, every parameter is known except Tf which is what I want to solve by doing this numerical method given the known parameters.
At time zero, for example
tao = A*Exp[((x*ah)/(R*Tk)) + ((1 - x)*ah/Subscript[T, fkminus1])] =
7.6*10^-38*
Exp[((0.49*342496)/(8.314*499.75)) + ((1 - 0.49)*342496/500)];
and then with the above tao we use it to calculate Tf at time 0 and then you can calculate tao at the next time, which allows you to calculate 'Tf' and the same time. The process repeats in an iterative way (which I don't know how to do in Mathematica) until we are able to plot for instance Tf vs T for a set of T. The set of T in this case goes from 500 to 350 (to be able to plot as in the figure). Notice that because the temperature set is already fixed between 500 and 350, the number of steps n is simply n = (T0 - Tfinal)/dt
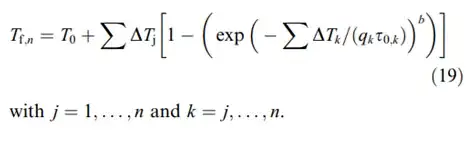





Tfis withk-1. I hope this is clear. – John Nov 26 '20 at 19:51taoat time 0 which allows you to calculateTf(which is what I want) at time 0 which in turn allows you to calculatetaoat the following time and this in turn allows you to calculateTfat the next following time and so.... Let me know if you need any further clarification – John Nov 26 '20 at 22:06T,kis a process temperature (for example if I heat soap in an oven, it will be the temperature I set the oven) whileTf,kis formally known as the "fictive temperature" and can be regarded as being similar to the "glass transition temperature" or the temperature at which a material goes from a hard to a glassy solid (hence it depends on the material or in the soap example where the soap will change to another state to due to process temperature). I hope that's clear and let me know if you have any other quesiton – John Nov 27 '20 at 02:05