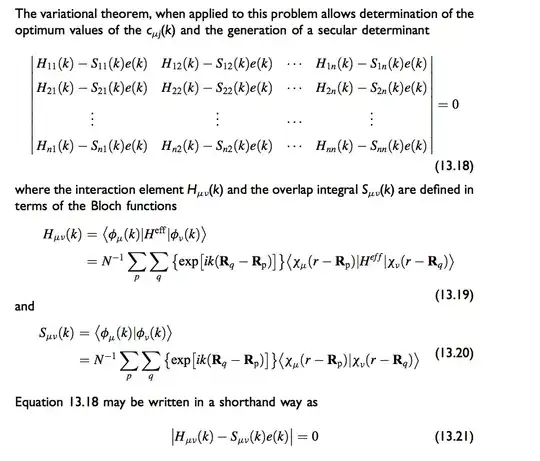
I've been reading Albright's Orbital Interactions in Chemistry. In the chapter on solids, he provided a general approach to find the band structure of a solid state system
Now if we are to model a one dimensional hydrogen chain, let $|1s(r)\rangle$ denote the $1s$ orbital centered at $r$. The band energy at each $k$ point will just be $e(k)=\frac{H_{11}(k)}{S_{11}(k)}$ according to the secular determinant. I then use a first neighbor interaction simplification, so $H_{11}(k)=\langle 1s(r)|H_{eff}|1s(r)\rangle+2\cdot\exp(ika)\langle 1s(r)|H_{eff}|1s(r-a)\rangle$ and $S_{11}=1+2\cdot\exp(ika)\langle1s(r)|1s(r-a)\rangle$.
It's worth noticing that assuming orthogonality of neighboring orbitals will set $S_{11}=1$, then $\langle 1s(r)|H_{eff}|1s(r-a) \rangle$ and $\langle 1s(r)|H_{eff}|1s(r)\rangle$ are just analogous to $\beta$ and $\alpha$ parameters in the simple Huckel model. But for the sake of realism I’m considering overlaps(the textbooks is also doing that) That is why the plots below show an asymmetric split with regard to hydrogen 1s ground state energy.
So far so good. I can even get a quick numerical estimate of $H_{11}$ using the extended Huckel approximation because then I just need to know the overlap integral between neighboring $1s$ orbitals. However, when I plot the result in Matlab it's different from the plot in the textbook:
Especially, the energy at the edge of the Brillouin zone is too positive. Anybody knows why that might happen? Thanks!
PS: I have to cross post this from Chemistry stackexchange because I'm feeling like this is more of a computational problem I have...