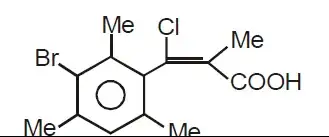
Is the compound in the picture chiral or achiral?
The answer in practice is: it depends. You say that there would be free rotation along the phenyl–vinyl bond. However, every adjacent carbon atom — the 2- and 6-positions of the phenyl ring and the 1-position of the double bond — are substituted. For free rotation to occur, these substituents must pass each other. Since they are all bulky, though, free rotation is very effectively hindered. A planar rotamer with full π conjugation between the phenyl ring and the double bond is not populated at any extend which can be shown by the NMR shifts of the corresponding carbon atoms (and could also be shown if the methyl group on the double bond were a proton, because it’s shift would be vastly different from one in styryl conjugation).
Under low enough temperatures, the compound you have been given will indeed form to separable enantiomers due to the bromine atom which destroys the molecule’s symmetry. These are examples of axial chirality; if the chlorine atom is above the phenyl ring’s plane, it is (aR), if it is below, the compound is (aS). The two enantiomers can be separated, e.g. by chiral HPLC, and they will show optical activity.
At higher temperatures, thermal energy will be enough to overcome the rotation barrier and an optically active single enantiomer will racemise. This temperature is typically above room temperature for a system such as your’s but well below the temperature required for alkene (E/Z) isomerisation.
This concept is called atropisomerism and is also the reason why BINOL and BINAP are chiral.