What started as this question http://chemistry.stackexchange.com/questions/67756/is-it-possible-to-use-the-school-supplied-algorithm-to-build-the-lewis-diagram-o continued with an email from the teacher, where they confirmed that the octet rule has to be followed and also mentioned that the oxidation number of iodine in iodate is 7, not 5 as I believe.
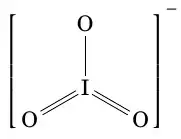
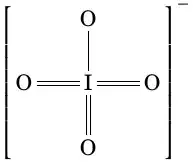
My thinking is based on the fact that oxygen is always 2, therefore iodine has 3*2-1=5. Two double bonds and one single bond with oxygen are present in iodate ion.
Iodine has oxidation number 7 in periodate ion as 4*2-1=7.
Where is my error?
EDIT:
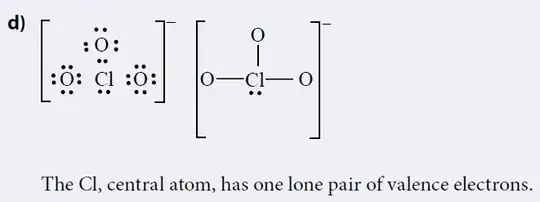
More back and forth with the teacher, and they pointed me at an example of how they think $\ce{[ClO3]-}$ should look in a Lewis diagram:
According to the teacher, both chlorate they have in the course and iodate in the assignment would have to follow the exact same steps when building Lewis diagram.
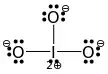
I have an issue with this methodology, as two $O$ are somehow missing their 2nd bonds. This is absurd and does not jive with any of the textbooks I studied. How should I explain this to the teacher, who does not seem to understand basic arithmetic?
httpsmy a bot edit in line withhttpsbeing used across SE and images should always include an image-describing alt-text stating what they are about. – Jan Jun 13 '17 at 13:35