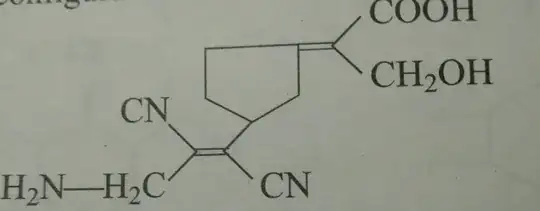
In the above we need to find the configuration of the two double bonds whether (E) or (Z) according to me the one with the cyanides should be (E) and the other (E) however my book say that the correct answers are (E,Z) (in the same order as above) . Please explain how ?
Asked
Active
Viewed 354 times
0
-
5Please show your precedence order; that way it would be easier to spot an error, if any. – Ivan Neretin Apr 05 '17 at 10:24
-
1Are you familiar with the Cahn-ingold-Prolog sequences? – xavier_fakerat Apr 05 '17 at 11:06
-
1I get (E) for both alkenes as well. – Ben Norris Apr 05 '17 at 18:34
-
Chemdraw gives (E) for both alkenes also. – jerepierre Apr 05 '17 at 21:52
1 Answers
1
You are correct; the answer in your book is wrong. I get (E) for both alkenes.
For the alkene on the upper right:
- $\ce{COOH}$ has precedence over $\ce{CH2OH}$ because the first carbon atom is bonded to $\mathrm{ \{O,O,O\}}$ and the second is bonded to $\mathrm{\{O,H,H\}}$.
- Clockwise around the ring has precedence over counterclockwise. While both initially are $\mathrm{\{C,H,H\}}$, the next carbon atom clockwise is $\mathrm{\{C,C,H\}}$ while the next carbon atom counterclockwise is $\mathrm{\{C,H,H\}}$.
For the alkene on the lower left, the nitrile groups win in both cases:
- On the left: $\mathrm{\{N,N,N\}}$ versus $\mathrm{\{N,H,H\}}$
- On the right: $\mathrm{\{N,N,N\}}$ versus $\mathrm{\{C,C,H\}}$
Ben Norris
- 42,831
- 8
- 123
- 181
-
I was thinking along those line, but wanted to confirm isn't it priotities decrease clockwise? – xavier_fakerat Apr 05 '17 at 19:06
-
Around the ring from the alkene in the upper right, clockwise > counterclockwise since CCH > CHH at the second carbon atom out. – Ben Norris Apr 06 '17 at 01:40