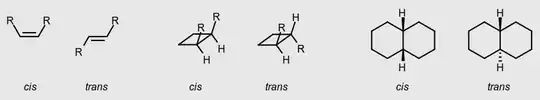
Stereoisomeric olefins, cycloalkanes, etc. which differ in the positions of groups relative to a reference plane: in the cis-isomer the atoms are on the same side, in the trans-isomer they are on opposite sides. An obsolete synonym, for which the usage is strongly discouraged, is geometric isomerism.
According to the IUPAC Gold Book:
geometric isomerism [obsolete]
(DOI: 10.1351/goldbook.G02620)
Obsolete synonym for cis-trans isomerism. (Usage strongly discouraged).
cis-trans isomers
(DOI: 10.1351/goldbook.C01093)
Stereoisomeric olefins or cycloalkanes (or hetero-analogues) which differ in the positions of atoms (or groups) relative to a reference plane: in the cis-isomer the atoms are on the same side, in the trans-isomer they are on opposite sides.