This is a problem of counting lone electron pairs as part of the delocalized pi system. In pyrrole you count the lone pair on the nitrogen, in pyridine you don't. What's the difference?
In pyrrole you have the lone pair left over after you have given every ring a ligand (the outer hydrogen atoms). With the sigma bonding requirements thus satisfied, the lone pair goes into the pi system. In pyridine the nitrogen is missing its ligand, so the lone pair must replace that in the plane of the ring and can't go into the pi system.
Which situation corresponds to your compound depends on what compound is meant, and there seems to be confusion on this point. Cyclopropenylidene ($\ce{C3H2}$) and cyclopropenyl anion ($\ce{C3H3^-}$) differ only by a carbon-hydrogen bond, and the shorthand method commonly used to render organic structures thus fails to distinguish them. The problem statement should be checked to ascertain which of these compounds is meant because they give different outcomes, and the answer key is right for only one of them.
If it is cyclopropenylidene
In cyclopropenylidene, the carbon with the lone pair is missing a ligand like pyridine, so the lone pair has to replace the missing ligand in the plane of the ring. That leaves two pi electrons to be delocalized which makes the ring aromatic. Cyclopropenylidene is not only aromatic by the electron count, it is a sufficiently preferred three-carbon structure to be seen in interstellar space.
If it is cyclopropenyl anion
Cyclopropenyl anion has all the ligands in place, so the lone pair goes into the pi system like pyrrole. Unfortunately in this case, adding that lone pair gets you four pi electrons in the ring and that's bad. In fact it turns out to be really had, even worse than larger (and thus more flexible) formally antiaromatic rings like cyclooctatetraene.
Cyclopropenyl anion goes through some serious contortions to get rid of this antiaromaticity. See this question.
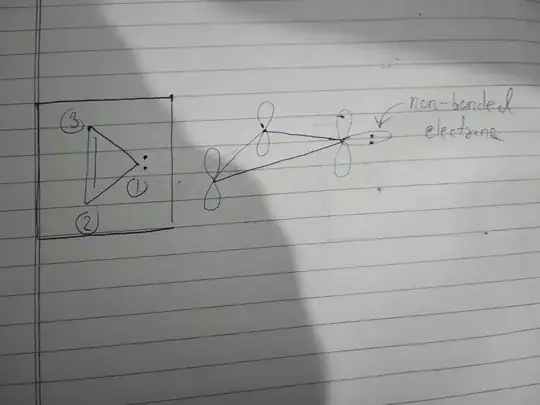 Here's a compound which we have to tell whether it's aromatic or not.
Here's a compound which we have to tell whether it's aromatic or not.