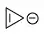When you have a ring that has $4n$ π electrons it's anti-aromatic if you force it into a fully conjugated cycle.
Often such rings find a way to break the unfavorable (or at least, less favored) anti-aromatic conjugation. Large ones like cyclooctatetraene are likely to bend so all ring atoms are no longer in the same plane. Small ones like the cyclopropenyl anion shown here can't bend the ring out of planarity easily (or do it at all with only three atoms), but they can still break the conjugation by using unequal bond lengths or moving a ligand out of the plane giving that ring atom a pyramidal bonding geometry.
The pyramidal bonding geometry seems to be what cyclopropenyl anion actually does. A computational result is shown by Kass [1]; the top carbon atom has its bonds directed towards the observer indicating a pyramidal geometry (see below, from https://comporgchem.com/blog/archives/2987 and [1]):

Reference
1.
Kass, S. R. "Cyclopropenyl Anion: An Energetically Nonaromatic Ion," J. Org. Chem. 2013, 78, 7370-7372, https://doi.org/10.1021/jo401350m.