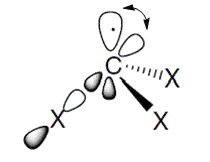Having three σ bonds in a similar manner to $\ce{CH3^·}$ free radical, $\ce{CF3^·}$ should also have $\mathrm{sp^2}$ hybridisation. However, if we look at its shape, it is pyramidal and not planar like $\ce{CH3^·}$ free radical (which is $\mathrm{sp^2}$-hybridised), which signifies that $\ce{CF3^·}$ should have $\mathrm{sp^3}$ hybridisation.
But how is this possible because the three σ-bonds will bond with three hybrid orbitals? Where does the third p-orbital come?
If its hybridisation is $\mathrm{sp^3},$ then why is it?